Washington
Magnolia Medical's New Blood Culture Collection Technology Enables Children's Hospitals to Reduce Contamination Rates to Zero
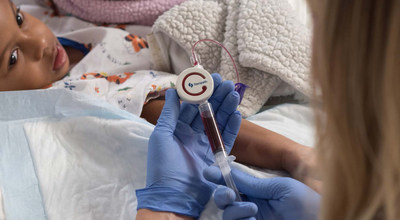
SEATTLE, July 29, 2021 /PRNewswire/ -- Magnolia Medical Technologies, Inc., inventors of Steripath®, the only FDA 510(k)-cleared device platform indicated to reduce blood culture contamination1 for sepsis testing, announces the commercial availability of its new Steripath® Micro Initial Specimen Diversion Device® designed for children's hospitals. Use of the new Steripath Micro device in two leading children's hospitals has shown a zero percent contamination rate over the last three months.
"Working with children's hospitals across the country, we are excited to launch Steripath Micro, the first technology solution specifically designed to reduce blood culture contamination for our most vulnerable patient populations," said Greg Bullington, CEO and Co-Founder of Magnolia Medical. "We are proud of the exhaustive clinical results we have generated with our Steripath platform proving that false positive blood culture results used to diagnose sepsis can be eliminated with our technology. It's very encouraging to see a zero percent contamination rate demonstrated with our new Steripath Micro device."
Blood cultures are an essential diagnostic tool and remain the standard of care for detecting microorganisms in a patient's bloodstream.2,3 As one of the most critical microbiology tests, blood cultures are used to confirm or rule out life-threatening bloodstream infections, including sepsis, and provide necessary information to optimize treatment.
Despite the clinical importance, false positive results are common. Historically, the testing process has not accounted for the realities of dislodged skin plugs and fragments containing viable microorganisms from venipuncture. Consequently, 30 to over 50 percent of blood cultures can be contaminated, producing a false-positive test result.4 These false-positive results trigger risk of overuse of toxic antibiotics, re-tests, delayed appropriate treatment, additional interventions, extended hospital stays and higher costs for the patient and the hospital.2 The associated cost of a contamination event at a children's hospital has been reported as a median of $8,720.5 In addition, false positives can contribute to resistant bacterial strains, long-term societal effects, and increased healthcare burden and costs.6
"We are encouraged by the early results we've seen so far in the reduction of blood culture contaminants since using the Steripath Micro device and we're eager to see more data over the next several months ," said Dr. Rick Place, Pediatric Medical Director, Department of Emergency Medicine.
Steripath Micro is FDA 510(k)-cleared with the specific indication to reduce blood culture contamination.1 For children's hospitals, this means that patients with suspected bloodstream infections, such as sepsis, can now receive an accurate diagnosis, enabling precise and timely treatment. Steripath Micro is a small, lightweight, easy-to-use device that can standardize the blood culture collection process, providing immediate and sustainable reduction in contamination rates which can improve patient outcomes and decrease healthcare burden from sepsis misdiagnoses.
About Magnolia Medical
Magnolia Medical Technologies develops, manufactures, and markets innovative blood and bodily fluid collection devices to facilitate significant improvements in the accuracy, consistency, and predictability of critical laboratory tests. Magnolia Medical invented and patented the Initial Specimen Diversion Technique (ISDT) and Device (ISDD®) for blood culture collection and contamination prevention. The company has amassed an intellectual property portfolio including more than 80 issued method, apparatus, and design patents with more than 70 additional patent applications pending. For more information, visit www.magnolia-medical.com.
References:
1. Indicated for use as a blood collection system that diverts and sequesters the initial specimen prior to collection of a subsequent test sample to reduce the frequency of blood culture contamination when contaminants are present in the initial blood sample compared to blood cultures drawn with standard procedure without manual diversion. 2. Lamy B, et al. How to optimize the use of blood cultures for the diagnosis of bloodstream infections? A state-of-the art. Front Microbiol. 2016;7:697 10.3389/fmicb.2016.00697 3. Doern, Gary V., et al. A comprehensive update on the problem of blood culture contamination and a discussion of methods for addressing the problem. Clinical Microbiology Reviews 33.1 (2019). 4. Garcia RA, et al. Multidisciplinary team review of best practices for collection and handling of blood cultures to determine effective interventions for increasing the yield of true-positive bacteremias, reducing contamination, and eliminating false-positive central line-associated bloodstream infections. Am J Infect Control. 2015;43(11):12221237pmid:26298636. 6. Gander RM, et.al. Impact of blood cultures drawn by phlebotomy on contamination rates and health care costs in a hospital emergency department. J Clin Microbiol. 2009;47(4):10211024pmid:19171686 7. Bates DW, et.al. Contaminant blood cultures and resource utilization. The true consequences of false-positive results. JAMA. 1991;265(3):365369pmid:1984535.
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/magnolia-medicals-new-blood-culture-collection-technology-enables-childrens-hospitals-to-reduce-contamination-rates-to-zero-301343971.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/magnolia-medicals-new-blood-culture-collection-technology-enables-childrens-hospitals-to-reduce-contamination-rates-to-zero-301343971.html
SOURCE Magnolia Medical Technologies